Bacterial attachment to the host surfaces is the first and key step in colonization, which may harm or benefit the host based on the natureof host-microbial relationship. Bacteria often assemble and use hair-like organelles known as pili or fimbriae on their cell surface to quickly and effectively mediate attachment. This initial recognition between the bacteria and host through surface molecules determines the tissue tropism and defines host range. Combating bacterial infections by targeting pili or pili-mediated interactions is recognized as a promising approach that may help overcome their ever-increasing repertoires of resistance mechanisms. This requires knowledge of how bacteria assemble pili and use them for adherence. In pathogens, the pili and their components have been studied and also recognized as virulence factors and good vaccine candidates because of their key role in pathogenesis and immunogenic properties.
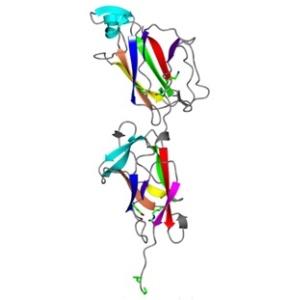
Interestingly, the beneficial or probiotic bacteria that benefit us also use pili to grab the human gut surface and compete for adhesion with harmful microbes, thus excluding them. Dr. Vengadesan Krishnan’s structural biology research group has begun structural investigations towards understanding how the beneficial bacteria have evolved to utilize pili to adapt the gut environment for survival. As part of their ongoing research, Dr. Krishnan’s group has recently discovered the crystal structure of a building block (backbone pilin) that forms the pilus fiber in the probiotic bacterium Lactobacillus rhamnosus GG. This study presents the first pilin structure from a beneficial bacteria or probiotics and provides new insights about pilus formation. A unique combination of isopeptide bonds and shape complementarity within and between the blocks allow this bacterium to assemble an elongated spring-like pilus fiber. This work contributes to the understanding of how a beneficial probiotic microbe assembles stable pili with the help of isopeptide bonds to withstand environmental shear forces and colonize the gut. The authors further show that the autocatalytic isospeptide bond formation takes place even in the absence of essential catalytic residue.
For more details, https://www.ncbi.nlm.nih.gov/pubmed/27349405

