 Aryl azides are considered as “green” reagents as most of their reactions involve only a benign loss of nitrogen. On the other hand, in the ‘Click’ reaction all the three nitrogen atoms are retained. Aryl azides are very reactive and many short lived intermediates are generated by both thermal and photochemical decomposition of aryl azides. Aryl nitrenes lead to a myriad of possible intermediates yielding products often accompanied by tarry products making it challenging to isolate and purify the products.
Aryl azides are considered as “green” reagents as most of their reactions involve only a benign loss of nitrogen. On the other hand, in the ‘Click’ reaction all the three nitrogen atoms are retained. Aryl azides are very reactive and many short lived intermediates are generated by both thermal and photochemical decomposition of aryl azides. Aryl nitrenes lead to a myriad of possible intermediates yielding products often accompanied by tarry products making it challenging to isolate and purify the products.
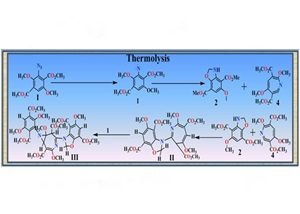
Thermolysis of azido dimethylsuccinylosuccinate was carried out to study the behavior of this new azide during thermolysis to compare the products obtained in our earlier work on thermolysis of ‘azido-m-hemipinate’, where ‘long-lived’ transients are presumably involved. The products obtained have been studied by various spectrometric & computational methods. Compound II, is ‘dimeric’ in nature formed by insertion of the nitrene intermediate into the adjacent o-methoxy group resulting in the formation of a nucleophilic oxazole ring which further adds to the 7-membered heterocumulene intermediate to give compound II. Compound III is ‘trimeric’ in nature and is presumably formed via the addition of the initial nitrene intermediate 1 to the double bond in compound II, resulting in the formation of the fused azidirine ring in compound III.
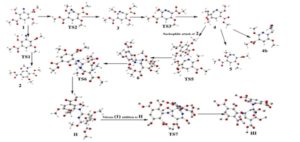
Calculated geometries [using SMD-B3LYP/6-31+(d,p) in chlorobenzene] of various intermediates involved in formation of Compound-II and compound-III (Hydrogen atoms from TS7 and compound-III have been removed for clarity).
DFT [Gaussian 09; B3LYp(d,p)] results rationalized the formation of compounds II &III from the stable intermediates 2 & 4. Further DFT results suggest that the reaction between these two intermediates is more favorable to reaction of 4 with pyridyl carbene and the carbene intermediate, which corroborates that such products were not formed during the thermolysis. The compounds characterized in this study are previously unknown and Structure Activity Relationship (S. A. R.) studies, with both wet and computational methods, could lead to potentially active new chemical entities (N. C. E.’s) as drug candidates.
Reference for full article:
Eswaran SV, et al., Tetrahedron (2017), Volume 73, Issue 35, 31 August 2017, Pages 5280-5288; http://dx.doi.org/10.1016/j.tet.2017.07.024.


