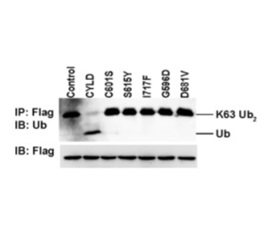
 Tumor suppressor cylindromatosis protein (CYLD), which specifically cleaves lysine
Tumor suppressor cylindromatosis protein (CYLD), which specifically cleaves lysine
63-linked ubiquitin chain from its substrate molecules, contributes to myriad of
important cellular events including cellular differentiation, oncogenesis, DNA repair
and cell cycle control. It is a ubiquitously expressed protein, which negatively
regulates NF-kB and JNK signaling pathways and mediates caspase dependent
apoptosis through RIP1 deubiqutination. Germline mutations in CYLD are associated
with a rare, hypertrophic skin cancer, termed Familial Cylindromatosis. Catalogue of
Somatic Mutations in Cancer database ensembles accumulating CYLD point
mutations in multiple benign and malignant tumors. However, the functional role of
CYLD mutations and their association with cancer progression remains elusive. We
have shown that cancer associated mutations impose structural alteration in CYLD
which impairs its binding to K63 ubiquitin chain. The mutant proteins required a
higher concentration of urea to unfold and there is a significant increase in the melting
temperature profiles of CYLD mutants. ANS fluorescence experiments display high
content of exposed hydrophobic patches in CYLD WT protein and subsequent
mutants. All the cancer mutants of CYLD displayed a notable structural
destabilization effect. We conclude that the loss of CYLD catalytic activity
potentiates its oncogenic gain of function through increased cell survival and
migration.
For full article: https://doi.org/10.1016/j.bbagen.2018.05.016


Fluoxetine Us Pharmacy can you get levitra cheap Sinequan Viagra Sale